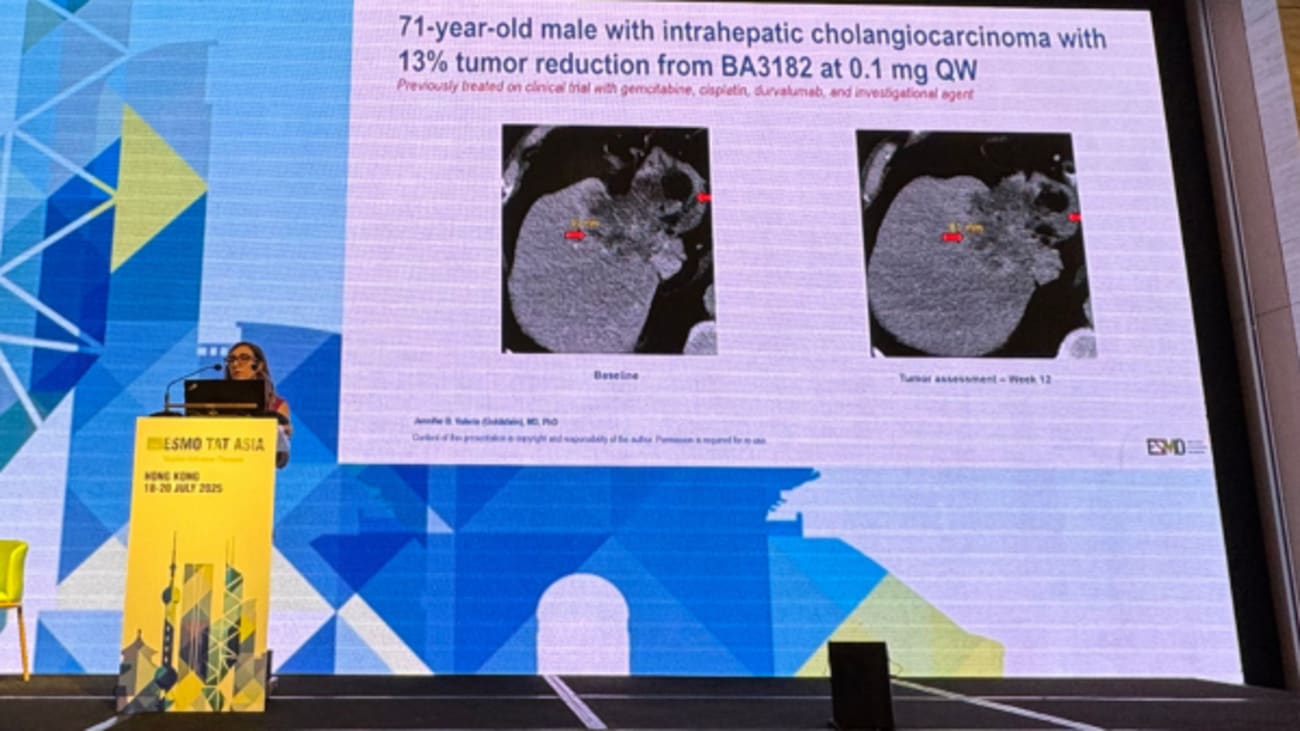

At the 2025 European Society for Medical Oncology Targeted Anticancer Therapies (ESMO TAT) Asia Congress this month, Jennifer B. Valerin, MD, PhD, associate clinical professor at the UCI Health Chao Family Comprehensive Cancer Center, presented early findings from a Phase 1 clinical trial investigating BA 3182, a conditionally active bispecific T cell engager (BiTe) therapy targeting EpCAM and activated T cells.
This clinical approach is designed to activate only in low pH tumor environments and early data shows that the drug is relatively well tolerated due to this conditional mechanism. Preliminary data also shows signs of prolonged disease stability, particularly in colorectal cancer, as this trial currently has 22 patients enrolled to date. As the principal investigator from UC Irvine, Valerin looks forward to sharing more efficacy data later this year.
